- India
- International
CBSE class 10th Science sample question paper
CBSE class 10 science sample paper to assess your preparation. Check important topics and marking scheme as per the new exam pattern by the board.
 CBSE board exams to begin from February 15 (Representational Image)
CBSE board exams to begin from February 15 (Representational Image)
CBSE class 10 science: The Central Board of Secondary Education (CBSE) will conduct class 10 science paper on March 4. Those who are appearing for the exam can check their preparedness by practising the following test paper prepared by Genesis Global School.
Before appear, students should ensure to map their timing accordingly. The exam is of 80 marks and is for three hours duration. Rest 20 marks will be awarded based on performance in the practical exam. To pass, candidates need to secure 33 per cent marks separately in theory and practical exams, as per the CBSE rule.
Read| How to prepare for Board exams 2020
SECTION A
Q1. Name a device that helps to maintain a potential difference across a conductor. [1]
Q2. What change in colour is observed when white silver chloride is left exposed to sunlight? What type of chemical reaction is this? [1]
Q3. Answer question numbers 3.1 – 3.4 on the basis of your understanding of the following paragraph and the related studied concepts.
The ciliary muscles of eye control the curvature of the lens in the eye and hence can alter the effective focal length of the system. When the muscles are fully relaxed, the focal length is maximum. When the muscles have strained the curvature of lens increases (that means radius of curvature decreases) and focal length decreases. For a clear vision, the image must be on retina. The image distance is therefore fixed for clear vision and it equals the distance of retina from eye-lens. It is about 2.5 cm for a grown-up person.

A person can theoretically have a clear vision of objects situated at any large distance from the eye. The smallest distance at which a person can clearly see is related to minimum possible focal length, The ciliary muscles are most strained in this position. For an average grown-up person, a minimum distance of an object should be around 25 cm.
A person suffering from eye defects uses spectacles (Eyeglass). The function of lens of spectacles is to form the image of the objects within the range in which a person can see clearly. The image of the spectacle-lens becomes an object for eye-lens and whose image is formed on retina.
The number of spectacle-lens used for the remedy of eye defect is decided by the power of the lens required and the number of spectacle-lens is equal to the numerical value of the power of a lens with a sigh. For example power of the lens required is +3D (converging lens of focal length 100/3 cm) then the number of the lens will be +3.
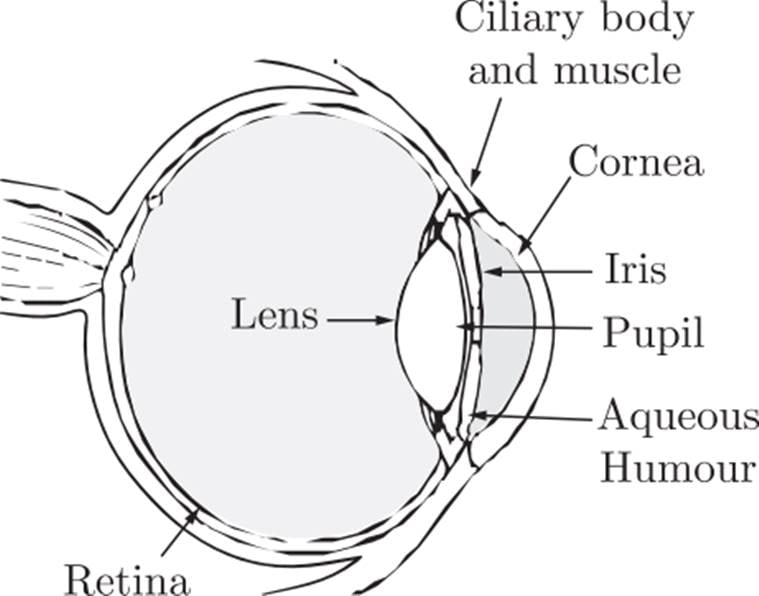
For all the calculations required you can use the lens formula and lens maker’s formula. Assume that the eye lens is equiconvex lens. Neglect the distance between the eye lens and the spectacle lens.
3.1 What do you mean by the ciliary muscles? [1]
3.2 What is the minimum focal length of the eye lens of a normal person? [1]
3.3 What is the maximum focal length of the eye lens of a normal person? [1]
3.4 A near-sighted man can clearly see an object only up-to a distance of 100 cm and not beyond this. What is the number of the spectacles lens necessary for the remedy of this defect? [1]
Q4. Question numbers 4.1 – 4.4 are based on the two tables given below. Study these tables related to blood pressure level and answer the question that follows:
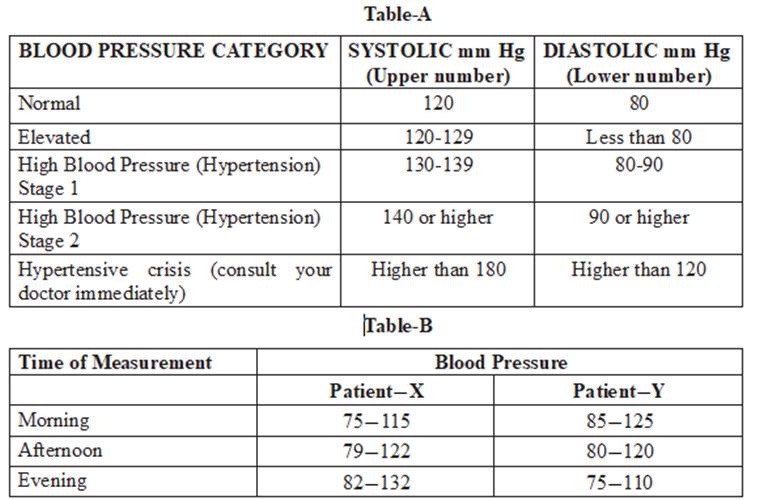
4.1 In table B, at which time patent-Y have ideal normal blood pressure? [1]
4.2 Identify the patient, which have hypertension stage-1 blood pressure? [1]
4.3 Which Diet is the best for high blood pressure patient? [1]
(3.a) Grain and fruits
(3.b) High fat dairy products
(3.c) Take large amount of sodium in diet
(3.d) All of the above
4.4 The ideal blood pressure measurement is [1]
(4.a) 80-120 mm Hg
(4.b) 85-125 mm Hg
(4.c) 90-150 mm Hg
(4.d) 95-100 mm Hg
Q5. The length of a wire is doubled. By what factor does the resistance change [1]
(a) 4 time as large (b) twice as large
(c) unchanged (d) half as large
OR
If a student while studying the dependence of current on the potential difference keeps the circuit closed for a long time to measure the current and potential difference, then
(a) ammeter’s zero error will change
(b) ammeter will give more reading
(c) voltmeter will show constantly higher readings
(d) resistor will get heated up and its value will change
Q6. A small electric lamp is placed at the focus of a convex lens. When the lamp is switched on, the lens will produce : [1]
(a) converging beam of light (b) parallel beam of light
(c) diverging beam of light (d) diffused beam of light
Q7. Before setting up an experiment to show that seeds release CO2 during respiration, the seeds should be : [1]
(a) dried completely (b) boiled to make then soft
(c) soaked in vinegar (d) kept moist till they germinate
Q8. A well-stained leaf peel mount, when observed under the high power of a microscope, shows nuclei in : [1]
(a) guard cells only
(b) epidermal cells only
(c) guard cells and epidermal cells
(d) guard cells, epidermal cells and stomata
OR
During germination of seed, water enter in seeds through
(a) hilum (b) micropyle
(c) raphe (d) cotyledon
Q9. Fe2 O3 + 2Al Al2 O3 + 2Fe
The above reaction is an example of a :
(a) combination reaction (b) double displacement reaction
(c) decomposition reaction (d) displacement reaction [1]
Q10. Ethanoic acid was added to sodium bicarbonate solution and the gas evolved was tested with a burning splinter Which one of the following four observations is correct? [1]
(a) The gas burns with a pop sound and the flame gets extinguished
(b) The gas does not burn but the splinter burns with a pop sound
(c) The flame extinguishes and the gas does not burn
(d) The gas burns with a blue flame and the splinter burns brightly
Q11. A colourless sample was tested with a strip of pH paper. The colour of the strip changed to green.

Q12. Beakers A, B and C contain zinc sulphate, silver nitrate and iron (II) sulphate solutions respectively. Copper pieces are added to each beaker. Blue colour will appear in case of [1]
(a) beaker A (b) beaker B
(c) beaker C (d) all the beakers
OR
A student puts one big iron nail each in four test tubes containing solutions of zinc sulphate, aluminium sulphate, copper sulphate and iron sulphate. A reddish-brown coating was observed only on the surface of iron nail which was put in the solution of
(a) zinc sulphate (b) iron sulphate
(c) copper sulphate (d) aluminium sulphate
For question numbers 13 and 14, two statements are given-one labelled Assertion (A) and the other labelled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c)and (d) as given below :
Q13. Assertion : Respiration in living beings is called exothermic reaction.
Reason : Respiration in living beings involves with absorption of heat energy [1]
(a) Both A and R are true and R is correct explanation of the assertion.
(b) Both A and R are true but R is not the correct explanation of the assertion.
(c) A is true but R is false.
(d) A is false but R is true.
Q14. Assertion: Copper is used to make hot water tanks and not steel.
Reason : Copper is a better conductor of heat than steel and it is fairly resistant to corrosion than steel. [1]
(a) Both A and R are true and R is correct explanation of the assertion.
(b) Both A and R are true but R is not the correct explanation of the assertion.
(c) A is true but R is false.
(d) A is false but R is true.
SECTION B
Q15. How do guard cells regulate opening and closing of stomatal pores? [3] Q16. 2 g of ferrous sulphate crystals were heated in a hard glass test tube and observations recorded.
a. What type of odour is observed on heating ferrous sulphate crystals?
b. Name the products obtained on heating ferrous sulphate crystals.
c. What type of reaction is taking place? [3]
OR
a. Why metals are not found in their free state generally?
b. If a strip of aluminium with scratched clean surface is dipped into an aqueous solution of copper sulphate for little time, surface of the strip becomes brownish. What is the reason for this? Write the balanced chemical equation for the reaction.
Q17. Write the chemical formula for washing soda. How may it be obtained from baking soda? Name an industrial use of washing soda other than washing clothes. [3]
Q18. Study the diagram given below:
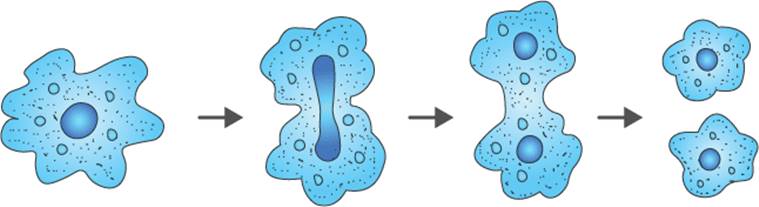
a. Identify the process.
b. Which organism uses the above method of reproduction?
c. How is the above method different from the process of fragmentation? [3]
OR
How do organisms, whether reproduced asexually or sexually maintain a constant chromosome number through several generations? Explain with the help of suitable example.
Q19. Out of the elements H(l), Be(4), Na(ll) and Mg(12).
a. Write the pair of elements having similar chemical properties.
b. State the group number of each pair,
c. Name one another element belonging to each of these groups. [3]
Q20. What are covalent compounds? Why are they different from ionic compounds? List their three characteristic properties. [3]
Q21. Why does the sun appear reddish early in the morning? Will this phenomenon be observed by an observer on the moon? Justify your answer with a reason. [3]
Q22. Suggest three contraceptive methods to control the size of the human population which is essential for the health and prosperity of a country. State the basic principle involved in each. [3]
Q23. Draw the following diagram, in which a ray of light is incident on a concave-convex mirror, on your answer sheet. Show the path of this ray, after reflection, in each case. [3]
Q24. a. State the function of ‘a fuse’ in a circuit. How is it connected in the domestic circuit?
b. An electric fuse of rating 3A is connected in a circuit in which an electric iron of power 1 kW is connected which operates at 220 V What would happen? Explain. [3]
OR
a. List the factors on which the resistance of a conductor in the shape of a wire depends.
b. Why are metals good conductors of electricity whereas glass is a bad conductor of electricity? Give reason.
c. Why are alloys commonly used in electrical heating devices? Give reason.
SECTION C
Q25. a. Give a chemical test to distinguish between saturated and unsaturated hydrocarbon.
b. Name the products formed when ethane burns in air. Write the balanced chemical equation for the reaction showing the types of energies liberated.
c. Why is reaction between methane and chlorine in the presence of sunlight considered a substitution reaction? [5]
OR
Account for the following.
a. Dry HCl gas does not change the colour of dry blue litmus paper
b. Antacid tablets are used by a person suffering from stomach pain.
c. Toothpaste is used for cleaning teeth.
Q26. Translate the following statements into chemical equations and then balance them.
a. Hydrogen gas combines with nitrogen gas to form ammonia gas.
b. Hydrogen sulphide gas burns in air to give water and sulphur dioxide gas.
c. Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
d. Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
e. Zinc metal reacts with dilute sulphuric acid to give zinc sulphate solution and hydrogen gas [5]
Q27. a. Draw a neat diagram of an excretory unit of a human kidney and label the following parts.
(i) Bowman’s capsule
(ii) Renal artery (iii)Glomerulus
(iv) Collecting duct
b. Give one advantage of having a large number of these highly coiled structures in our kidneys.
c. Mention any two substances which are selectively reabsorbed as the filtrate flows along the tubular part of this unit. [5]
Q28. a. Differentiate between pollen grain and ovule.
b. State in brief functions of the following parts of the human female reproductive system.
(i) Ovary
(ii) Fallopian Tube
(iii) Uterus [5]
OR
a. Differentiate between germination and fertilisation.
b. State in brief the functions of the following parts of the human male reproductive system:
(i) Scrotum (ii) Testes (iii) Vas deferens
Q29. A current of 1 ampere flows in a series circuit containing an electric lamp and a conductor of 5 W when connected to a 10 V battery. Calculate the resistance of the electric lamp. Now if a resistance of 10W is connected in parallel with this series combination, what change (if any) in current flowing through 5 W conductor and potential difference across the lamp will take place? Give reason. Draw circuit diagram. [5]
Q30. a. Define the following terms in the context of spherical mirrors:
(i) Pole
(ii) Centre of curvature (iii)Principal axis
(iv) Principal focus
b. Draw ray diagrams to show the principal focus of a :
(i) Concave mirror
(ii) Convex mirror
c. Consider the following diagram in which M is a mirror and P is an object and Q is its magnified image formed by the mirror
State the type of the mirror M and one characteristic property of the image Q . [5]
OR
a. Draw a ray diagram to show the formation of image by a convex lens when an object is placed in front of the lens between its optical centre and principal focus.
b. In the above ray diagram mark the object-distance u and the image-distance v with their proper signs (+ve or -ve as per the new Cartesian sign convention) and state how these distances are related to the focal length f of the convex lens in the case.
c. Find the power of a convex lens which forms a real, and inverted image of magnification -1 of an object placed at a distance of 20 cm from its optical centre.
Also Read| CBSE class 10 Mathematics sample paper
Meanwhile, CBSE has advised students to follow sample papers thoroughly and not the paper pattern in the curriculum. “Sample question papers include modifications introduced by the CBSE. It may please be noted that for 2020 examinations, sample question papers should be taken into consideration and not the design given in the curriculum,” read the release.
Apr 16: Latest News
- 01
- 02
- 03
- 04
- 05






































