- India
- International
Covaxin efficacy determined after two doses, says Bharat Biotech after Anil Vij contracts Covid-19
Bharat Biotech said as part of its trial, 50 per cent of the participants received the vaccine while the others were administered a placebo.
 Representational Image (File)
Representational Image (File)Hyderabad-based Bharat Biotech Saturday said the efficacy of its vaccine, Covaxin, for the novel coronavirus, could be determined only 14 days after a second dose. It said its clinical trials were based on a two-dose schedule, given 28 days apart.
The clarification from the company came hours after Haryana Health Minister Anil Vij, who had been administered one shot of Covaxin about two weeks ago as part of its clinical trials, said he tested positive for Covid-19.
Watch Video: How does intranasal vaccines for Covid-19 work?
“Covaxin clinical trials are based on a two-dose schedule, given 28 days apart. The vaccine efficacy will be determined 14 days post the second dose. Covaxin has been designed to be efficacious when subjects receive both doses,” the biotechnology company said in statement, reported news agency ANI.
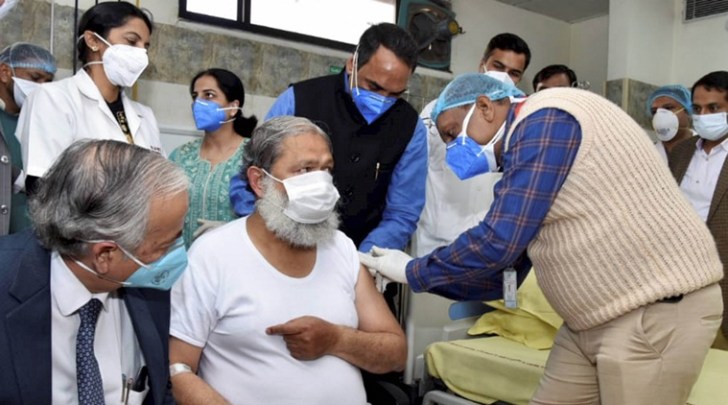 Anil Vij volunteers in the trials for potential coronavirus vaccine Covaxin, at Civil Hospital in Ambala district, Friday, Nov. 20, 2020. (PTI)
Anil Vij volunteers in the trials for potential coronavirus vaccine Covaxin, at Civil Hospital in Ambala district, Friday, Nov. 20, 2020. (PTI)
Explained Ideas: Is India ready to deliver Covid-19 vaccines? How equipped are we?
The company added that as part of the trial, 50 per cent of the participants received the vaccine while the others were administered a placebo. “The phase-3 trials are double-blinded and randomised, where 50 per cent of subjects (participants in the trial) receive vaccine & 50 per cent of subjects receive a placebo,” it said.

 Anil Vij was administered the Covaxin vaccine on November 20. (File Photo)
Anil Vij was administered the Covaxin vaccine on November 20. (File Photo)
Vij had volunteered to participate in Bharat Biotech’s human phase trials, in which over 25,000 persons were administered trial doses. On November 20, Vij was the first in Haryana to be administerd the shot. Besides him, over 400 persons from Haryana, including Rohtak PGIMS’ Vice Chancellor Dr. OP Kalra, participated in the trials.
Explained: What is emergency use authorisation firms are seeking for Covid-19 vaccines?
A team of Rohtak Post Graduate Institute of Medical Sciences had administered the vaccine to Vij at Ambala Cantonment’s civil hospital. His health and vitals were being monitored on a weekly basis ever since.
Bharat Biotech is developing the vaccine in collaboration with the National Institute of Virology (NIV) and the Indian Council of Medical Research (ICMR). It is among the several indigenous vaccines being developed in the country.
xApr 26: Latest News
- 01
- 02
- 03
- 04
- 05































