Successful synthesis of perovskite visible-light-absorbing semiconductor material
Narrow-gap semiconductors with the ability to use visible light have garnered significant interest thanks to their versatility. Now, scientists in Japan have developed and characterized a new semiconductor material for application in process components stimulated by light. The findings have, for the first time, suggested a new way to reduce the band gap in cheaper and non-toxic tin-based oxide semiconductors for efficient light-based applications.
Semiconductors that can exploit the omnipresent visible spectrum of light for different technological applications would serve as a boon to the material world. However, such semiconductors often do not come cheap and can often be toxic. Now, a group of material scientists from Tokyo Institute of Technology and Kyushu University have collaborated to develop a cheaper and non-toxic narrow-gap semiconductor material with potential "light-based" or photofunctional applications, according to a recent study published in Chemistry of Materials.
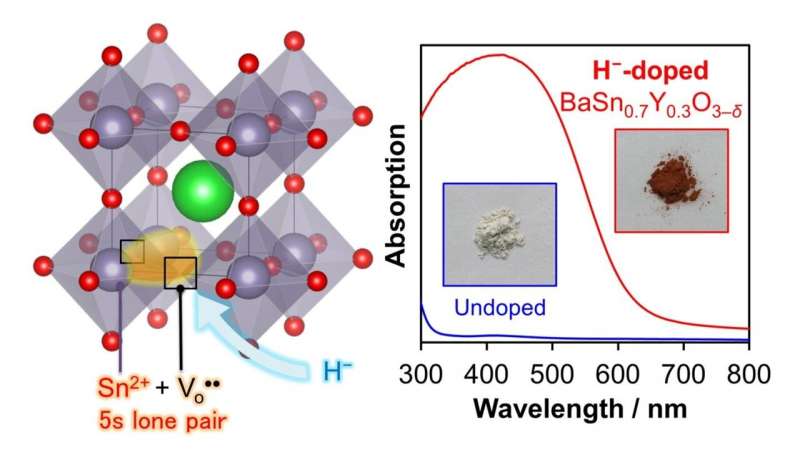
Tin-containing oxide semiconductors are cheaper than most semiconductor materials, but their photofunctional applications are constrained by a wide optical band gap. The aforementioned team of scientists, led by Dr. Kazuhiko Maeda, Associate Professor at the Department of Chemistry, Tokyo Institute of Technology, developed a perovskite-based semiconductor material that is free of toxic lead and can absorb a wide range of visible light (Figure 1). The team "doped," or intentionally introduced, hydride ions into the tin-containing semiconductor material. In doing so, they successfully reduced the band gap from 4 eV to 2 eV, due to the chemical reduction of the tin component that accompanied the hydride ion doping.
The scientists were also able to pinpoint a crucial tin reduction reaction in the semiconductor material through physicochemical measurements. This reduction leads to the generation of a "tin lone electron pair," whose different electronic states notably contribute to the visible light absorption of the material. They also attribute this desired property to the prior introduction of oxygen defects into the material. Highlighting the importance of the oxygen defects, Dr. Maeda, who is also a corresponding author of the study, explains, "The prior introduction of oxygen defects into BaSnO3 by Y3+ substitution for Sn4+ is also indispensable to realize a significant reduction of the band gap."
To confirm that the developed semiconductor material is indeed photofunctional, the scientists tested the applicability of the semiconductor material in a photoelectrode. They observed that the developed material gave a clear anodic photoresponse up to the expected 600 nm.
Speaking about the impact of the study, Dr. Katsuro Hayashi, Professor of the Faculty of Engineering, Kyushu University, and the other corresponding author of the study, says, "Overall, the study has enabled a giant leap in the development of a cheaper, non-toxic, narrow optical band gap, tin-containing semiconductor material for practical applications in solar cells, photocatalysis and pigments."
Thanks to the efforts of the researchers, we can expect significant advancements in the development of several more novel lead-free visible-light absorbing materials with myriad applications.
More information: Masashi Nakamura et al, Sn-Based Perovskite with a Wide Visible-Light Absorption Band Assisted by Hydride Doping, Chemistry of Materials (2021). DOI: 10.1021/acs.chemmater.1c00460
Journal information: Chemistry of Materials
Provided by Tokyo Institute of Technology