MEXICO CITY (Reuters) -Mexico plans to start a late-stage clinical trial this month for a COVID-19 vaccine candidate developed by China using similar technology to shots from Moderna and Pfizer, foreign minister Marcelo Ebrard said on Tuesday.
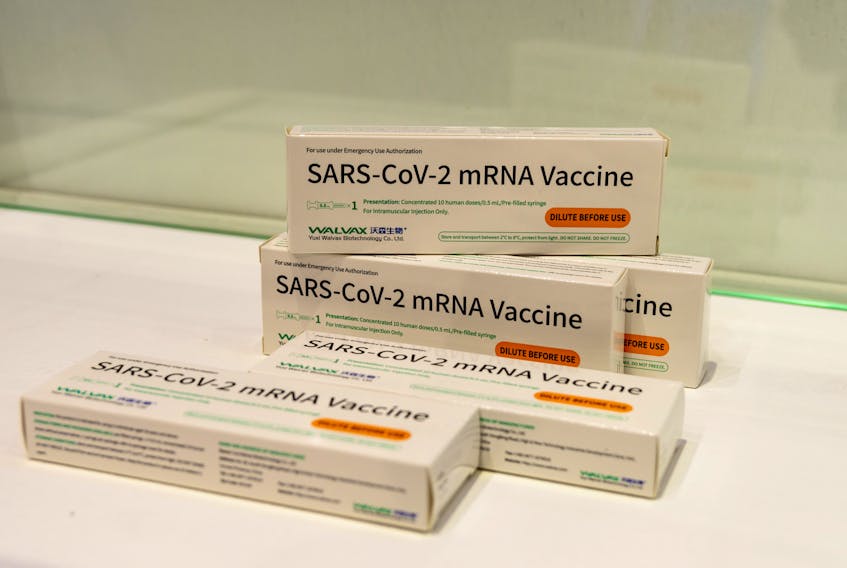
The phase III trial for the shot from China's Walvax Biotechnology using messenger RNA (mRNA) technology will start on May 30 and involve 6,000 volunteers, Ebrard said in a Tweet.
Walvax is working with the Academy of Military Science (AMS) and Suzhou Abogen Biosciences to jointly develop the shot known as ARCoV or ARCoVax, China's first mRNA vaccine to enter Phase III trials.
The shot could be stored at 2-8 degrees Celsius for six months, a much less stringent temperature requirement than shots from western rivals, an AMS researcher leading the vaccine project said in April during a presentation at a vaccine event.
Mexico has already received doses of other vaccines from China's Sinovac Biotech and CanSino Biologics, and planned to order shots made by Sinopharm.
China is currently using five domestically developed vaccines in its inoculation drive, although none use mRNA technology, which contains instructions for human cells to produce proteins that mimic part of the coronavirus.
(Reporting by Anthony Esposito in Mexico City, Roxanne Liu and Ryan Woo in Beijing; Editing by Andrew Heavens and Richard Pullin)