In a recent article posted to the medRxiv* preprint server, investigators in the United States illustrated that rebound of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can occur in some patients following molnupiravir and Paxlovid SARS-CoV-2 therapy.
 Study: COVID-19 rebound after Paxlovid and Molnupiravir during January-June 2022. Image Credit: NIAID
Study: COVID-19 rebound after Paxlovid and Molnupiravir during January-June 2022. Image Credit: NIAID

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Background
In December 2021, the Food and Drug Administration (FDA) approved Paxlovid (nirmatrelvir) and Lagevrio (molnupiravir) to treat mild-to-moderate SARS-CoV-2 infection in individuals at high risk of developing severe illness. However, recent case reports show that two to eight days after finishing a five-day treatment of Paxlovid, some patients suffered rebound CoV disease 2019 (COVID-19) and disease-related symptoms.
To inform the public about the possibility of COVID-19 rebound following Paxlovid therapy, the Centers for Disease Control and Prevention (CDC) recently released a Health Alert Network Health Advisory. Nevertheless, the prevalence of COVID-19 rebound in the general population, whether Paxlovid is the only medication that causes the rebound of SARS-CoV-2 infection, or if some people are more sensitive than others, remains uncertain.
About the study
The present work aimed to assess the relative risks and rates of COVID-19 rebound in SARS-CoV-2 patients who received Paxlovid or molnupiravir therapy and compare the traits of individuals who did and did not suffer the rebound of SARS-CoV-2 infection. The researchers used the TriNetX Analytics network system, a countrywide, multicenter database in the United States (US), to conduct a retrospective cohort analysis on the electronic health records (EHRs) of about 92 million individuals.
The trial population comprised 13,644 subjects aged ≥18 who became COVID-19-positive between 1 January and 8 June 2022. Further, within five days after contracting SARS-CoV-2, 11,270 and 2,374 of these individuals received Paxlovid and molnupiravir therapy, respectively. COVID-19 patients treated with both Paxlovid and molnupiravir were omitted from the study. The International Classification of Diseases, 10th revision (ICD-10) diagnosis code for COVID-19, i.e., U07.1, or laboratory-confirmed SARS-CoV-2 infection, was used to determine the status of COVID-19 among the participants.
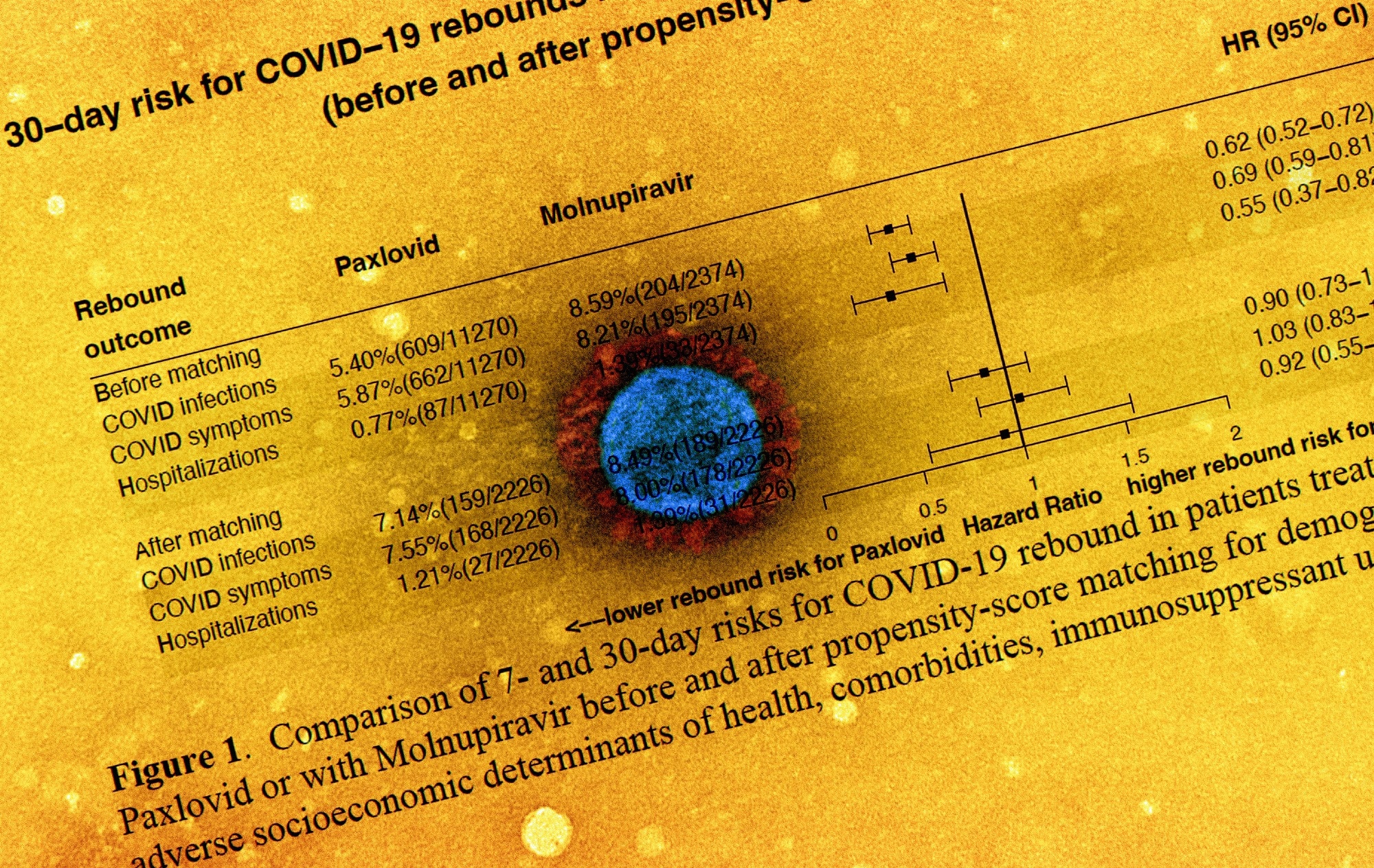
The research's primary outcomes and metrics were three kinds of COVID-19 rebound events (SARS-CoV-2 infections, COVID-19-linked symptoms, and SARS-CoV-2-associated hospitalizations) that happened two days following the last day of molnupiravir or Paxlovid therapy. Additionally, the 95% confidence interval (CI) and hazard ratios (HR) of the seven-day and 30-day likelihood for COVID-19 rebound among patients treated with Paxlovid and molnupiravir were calculated before and following propensity-score matching.
Results
Overall, the study results depicted that more COVID-19 patients were treated with Paxlovid relative to molnupiravir, which might be related to the two drugs' differing effectiveness in preventing SARS-CoV-2-linked hospitalizations or deaths among high-risk patients compared to placebo (88% for Paxlovid versus 30% for molnupiravir).
Across the two groups in the present study, the patients treated with Paxlovid were considerably different from those managed with molnupiravir, despite both medications being approved for use in SARS-CoV-2-infected individuals at high risk for severe COVID-19. The average age of Paxlovid-treated patients was 56 compared to 62 for molnupiravir-treated patients, and the initial cohort also had fewer co-existing health conditions. Furthermore, the Paxlovid arm comprised more Hispanics, women, Black, and Asian patients.
The rates of COVID-19 rebound following seven-day and 30-day Paxlovid treatment were 3.53% and 5.40%; 2.31% and 5.87%; and 0.44% and 0.77% for SARS-CoV-2 infection, COVID-19 symptoms, and SARS-CoV-2-related hospitalizations, respectively. Following molnupiravir therapy, the seven-day and 30-day SARS-CoV-2 rebound rates were 5.86% and 8.59%; 3.75% and 8.21%; and 0.84% and 1.39% for SARS-CoV-2 infection, SARS-CoV-2 symptoms, and COVID-19-linked hospitalizations, respectively.
There were no significant changes in the odds of COVID-19 rebound for SARS-CoV-2 infection between molnupiravir and Paxlovid following propensity-score matching: SARS-CoV-2 infection (HR 0.90 and 95% CI: 0.73-1.11), SARS-CoV-2 symptoms (HR: 1.03 and 95% CI: 0.83-1.27), or COVID-19-linked hospitalizations (HR: 0.92 and 95% CI: 0.56-1.55). Besides, compared to those without, patients with SARS-CoV-2 rebound had a statistically higher prevalence of underlying health issues.
Conclusions
The study findings indicated that SARS-CoV-2 rebound happened following treatment with both molnupiravir and Paxlovid, particularly in patients with pre-existing medical conditions. This shows that the COVID-19 rebound was not specific to Paxlovid and that the risks were comparable for both Paxlovid and molnupiravir. The team suggests that the rebound might be related to a chronic viral infection in some individuals who received either antiviral drug. In addition, the rates of SARS-CoV-2 rebound for both medications heightened over time following treatments.
The current results mandate ongoing monitoring of COVID-19 rebound following molnupiravir and Paxlovid treatments. The authors stated that further studies were required to identify the mechanisms underpinning SARS-CoV-2 rebounds and assess dosing and duration regimens that might stop them in susceptible patients.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.